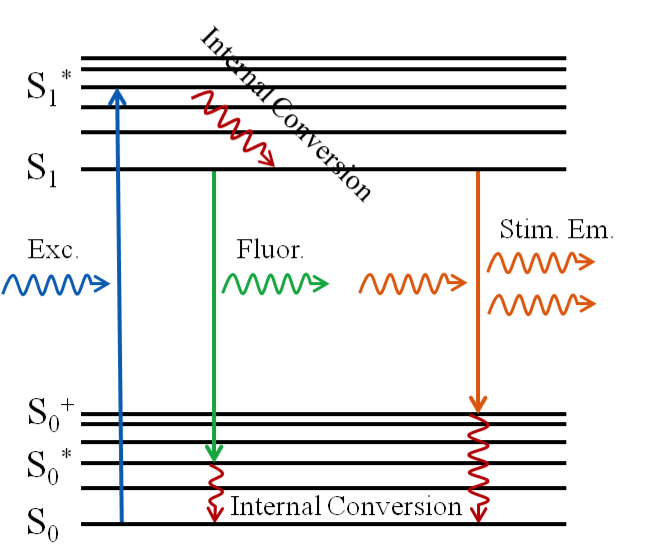
Stimulated Emission Depletion microscopy is a type of super resolution microscopy whose development is based on the principles of fluorescence and stimulated emission. When a photon enters with an energy equal to the difference in the energies of the electronic ground singlet state S0 and the first excited singlet state S1 the fluorophore is excited from the ground state to the excited state. This excitation is quickly followed by internal conversion which causes the electron to relax to the lowest vibrational excited state. Fluorescence is then obtained as the electron spontaneously decays to one of the vibrational states of the ground state, releasing a photon.
If instead, while the electron is still in the lowest vibrational state of S1, another photon comes in with an energy equal to that needed for an electronic transition to a vibrational state of S0 from the current state of the electron, then we end up with two photons that share the same properties (i.e. phase, polarization, etc…). This is stimulated emission. If the stimulated emission rate is much greater than the fluorescence emission rate, then the fluorescence is effectively eliminated.
In STED microscopy, two laser beams are used: excitation beam and depletion beam. The excitation beam (a 473nm CW beam in our case) is used to excite the fluorophores in our sample. Ignoring the depletion beam for the moment, we would expect the excitation point spread function (PSF) to look like a spot. If we now include the depletion beam (a 560nm CW beam for us) the PSF of this beam alone would appear to be donut-shaped, having been engineered by the phase plates in the microscope. There would be zero intensity in the center of this beam and non-zero intensity around this "donut hole". The overlap of these two PSFs would mean quenching of the fluorescence wherever the non-zero intensity of the depletion PSF overlaps the excitation PSF, yielding an image with a resolution of ≤50nm.
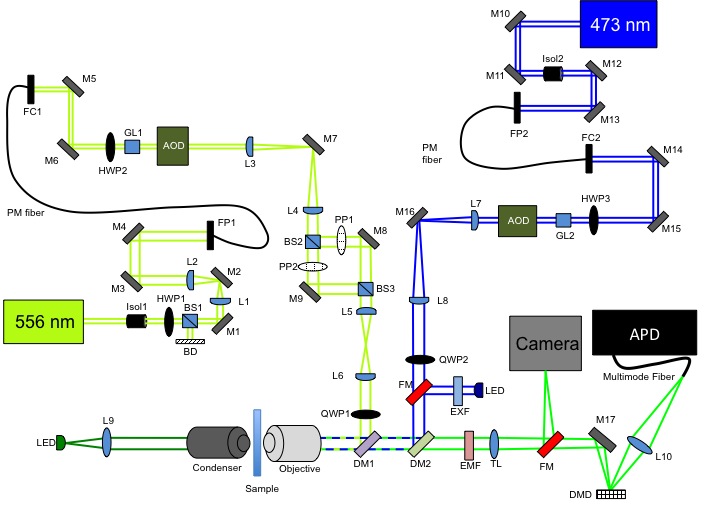
In our STED microscope, we are working to improve scanning rates by using acousto-optic deflectors (AOD) and a digital micromirror device (DMD). The AOD can scan at rates up to 100kHz and it would allow for rapid access to random locations within the sample which would mean less time spent on scanning uninteresting areas within the sample. Incorporating AODs would require a movable pinhole and thus a DMD would function as a movable pinhole. The micromirror device can be programmed to adjust the mirrors to be positioned at angles of ±10º. In this way, out of focus light will reflect off of the -10º mirrors (or +10º) and in focus light will reflect off of the +10º mirrors (or - 10º) into a detector or camera. Below is a diagram of our STED microscope.